Global high-mix volume high-speed PCBA manufacturer
9:00 -18:00, Mon. - Fri. (GMT+8)
9:00 -12:00, Sat. (GMT+8)
(Except Chinese public holidays)
Global high-mix volume high-speed PCBA manufacturer
9:00 -18:00, Mon. - Fri. (GMT+8)
9:00 -12:00, Sat. (GMT+8)
(Except Chinese public holidays)
HomePage > Blog > Knowledge Base > Medical PCBA: A Comprehensive Guide to Medical PCB Assembly
In the ever-evolving world of healthcare, technology plays a pivotal role in improving patient outcomes and advancing medical research. One of the most critical components behind this technological revolution is the printed circuit board assembly (PCBA) used in medical devices. Medical PCBAs are essential in powering various life-saving devices, from diagnostic machines to implantable devices, ensuring that they function with precision and reliability.
This blog will delve into the intricacies of medical PCBA, exploring its applications, design considerations, manufacturing processes, and future prospects. By understanding the role of medical PCBAs, readers can gain a deeper appreciation of how these components contribute to the success of modern medical technologies.
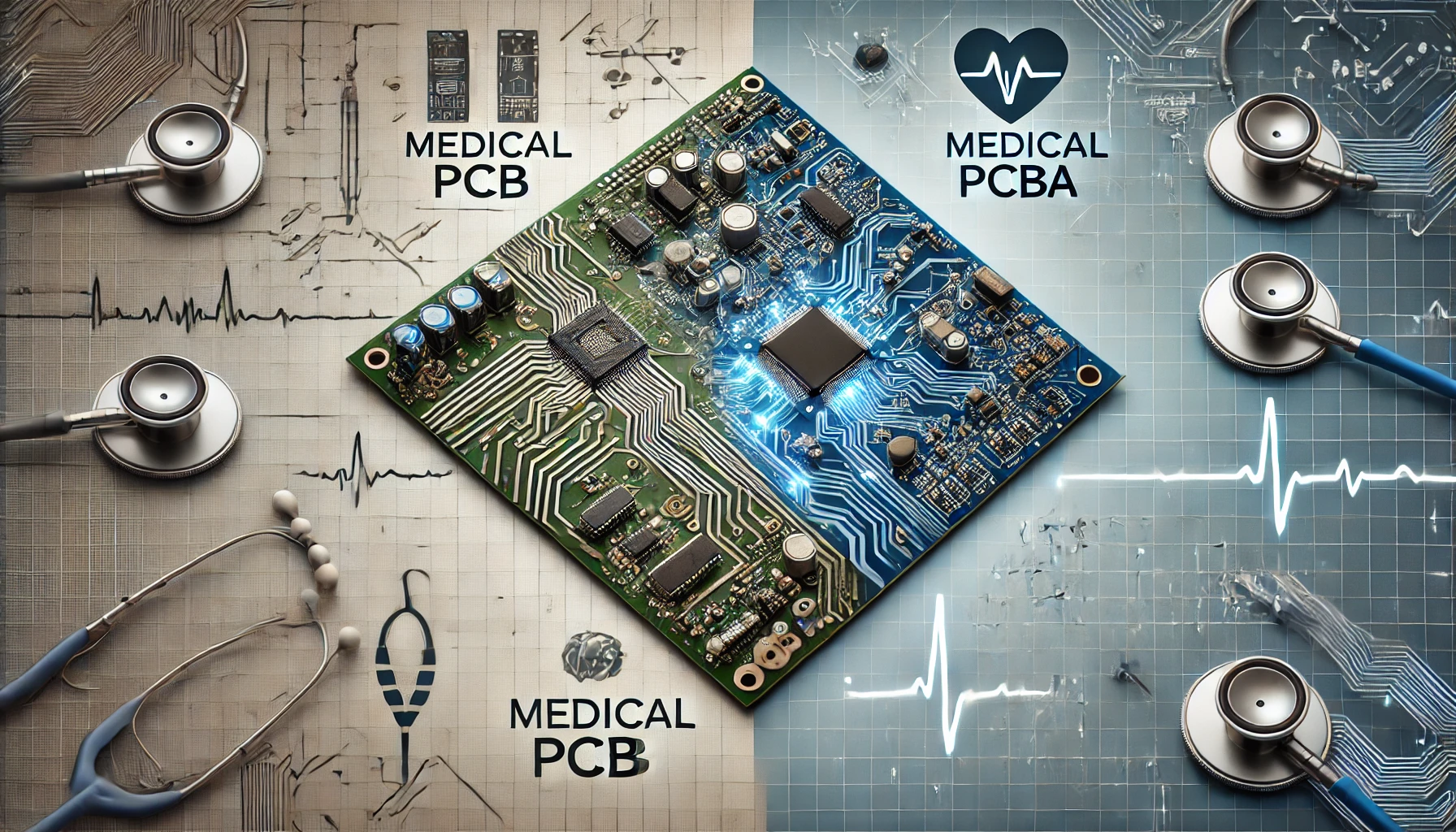
Before diving into the specifics of medical PCBA, it's essential to distinguish between a medical PCB and a medical PCBA.
Medical PCB (Printed Circuit Board): This refers to the bare board itself, which is made from non-conductive materials like fiberglass or plastic and contains conductive pathways etched from copper sheets. These pathways allow electronic components to communicate with each other.
Medical PCBA (Printed Circuit Board Assembly): This is the next step in the process, where components such as resistors, capacitors, and microchips are mounted onto the PCB to create a fully functional electronic circuit.
The primary difference between the two lies in their functionality: while a PCB is just the baseboard, a PCBA is a complete assembly that can be integrated into medical devices.
Medical PCBs are integral to the functionality of medical devices. They serve as the backbone for electronic circuits that power everything from diagnostic imaging systems like MRI machines to wearable health monitors and implantable devices like pacemakers. The role of these boards is critical because they ensure that medical devices operate with high precision and reliability—two factors that are non-negotiable in healthcare applications.
Medical devices are becoming increasingly sophisticated, requiring advanced PCBAs to meet stringent performance standards. Some examples of medical devices that rely on PCBs include:
Diagnostic Equipment: Devices such as MRI scanners, CT machines, and ultrasound systems depend on high-performance PCBAs for accurate image processing and data analysis.
Monitoring Devices: Wearable health monitors (e.g., heart rate monitors, glucose sensors) use compact and highly reliable PCBAs to provide continuous monitoring of vital signs.
Therapeutic Devices: Devices like infusion pumps and defibrillators rely on precise PCBAs to deliver accurate doses or life-saving interventions.
Implantable Devices: Pacemakers, cochlear implants, and neurostimulators require miniaturized yet highly reliable PCBAs that can function within the human body under strict safety standards.
These examples highlight how indispensable PCBs are in enabling advanced functionalities in modern healthcare technologies.
Designing a medical PCB requires careful consideration due to the unique demands of healthcare applications. Factors such as precision, reliability, and compliance with regulatory standards must be prioritized during the design phase.
One of the major challenges in designing medical PCBs is achieving high levels of complexity while maintaining precision. Medical devices often need to be compact yet powerful, which requires miniaturization without compromising performance. For instance:
Miniaturization: Many medical devices need to be small enough for portability or implantation within the body. This requires designers to pack more functionality into smaller spaces while ensuring that heat dissipation and signal integrity are maintained.
High Reliability: Given that these devices often operate in life-critical environments, there is no room for error. Designers must ensure that every component functions reliably under various conditions.
The selection of components for medical PCBs is another crucial aspect of design. Components must meet rigorous standards for durability, performance, and safety:
Durability: Components must withstand harsh environments such as extreme temperatures or exposure to bodily fluids.
Performance: High-performance components ensure that medical devices operate accurately and efficiently.
Compliance: Components must adhere to regulatory standards such as ISO 13485, which governs quality management systems for medical device manufacturing.
The manufacturing process for medical PCBs involves several critical steps designed to ensure quality and reliability.
The assembly process typically follows these steps:
1. Designing the Layout: The first step involves creating a schematic layout that defines where each component will be placed on the board.
2. Fabricating the PCB: The bare board is manufactured by etching copper onto non-conductive substrates.
3. Component Placement: Components are placed onto the board using automated machines.
4. Soldering: The components are soldered onto the board using techniques like reflow soldering or wave soldering.
5. Inspection & Testing: The assembled board undergoes rigorous testing to ensure it meets performance standards.
Two common techniques used in assembling medical PCBs are Surface Mount Technology (SMT) and Through-Hole Technology (THT).
SMT (Surface Mount Technology):
- In SMT, components are mounted directly onto the surface of the PCB without drilling holes.
- It allows for smaller component sizes and higher circuit densities.
- SMT is ideal for miniaturized devices like wearable health monitors.
THT (Through-Hole Technology):
- In THT, component leads are inserted into holes drilled through the board and soldered on the opposite side.
- THT provides stronger mechanical bonds but requires more space than SMT.
- It is often used in larger devices where durability is prioritized over size.
Given their critical role in healthcare applications, medical PCBAs undergo stringent quality control procedures:
Automated Optical Inspection (AOI): This technique uses cameras to inspect boards for defects such as misaligned components or soldering issues.
In-Circuit Testing (ICT): ICT checks individual components on an assembled board to ensure they function correctly.
Burn-In Testing: Boards are subjected to extreme conditions (e.g., high temperatures) to test their durability over time.
These testing methods help ensure that each board meets strict regulatory requirements before being integrated into a medical device.
The future of medical PCBA looks promising as new technologies continue to emerge:
Wearable Health Tech: As wearable health monitors become more prevalent, there will be increasing demand for compact yet powerful PCBAs that can handle real-time data processing.
Implantable Devices: Advances in miniaturization will allow for more sophisticated implantable devices capable of monitoring or treating various conditions from within the body.
AI Integration: Artificial intelligence will likely play a role in optimizing both device performance and manufacturing processes by enabling smarter diagnostics and predictive maintenance.
When it comes to sourcing medical PCBAs, finding a reliable manufacturer is crucial due to stringent industry regulations like ISO 13485 certification. A trustworthy manufacturer should offer:
- Proven experience in producing high-quality medical boards
- Compliance with international standards
- Advanced testing capabilities

Assembly Enquiry
Instant Quote
Phone contact

+86-755-27218592
In addition, we've prepared a Help Center. We recommend checking it before reaching out, as your question and its answer may already be clearly explained there.
Wechat Support

In addition, we've prepared a Help Center. We recommend checking it before reaching out, as your question and its answer may already be clearly explained there.
WhatsApp Support

In addition, we've prepared a Help Center. We recommend checking it before reaching out, as your question and its answer may already be clearly explained there.
